A spherical balloon of 21 cm dimeter is to be filled up with H2 at NTP from a cylinder containing the gas at 20 atm at 27o The cylinder can hold 2.82 litre of water at NTP. Calculate the number of balloons that can be filled up.
a) 20
b) 10
c) 15
d) 60
A 20 g chunk of dry ice is placed in an empty 0.75 litre bottle and tightly closed. The final pressure in the bottle after all CO2 has been evaporated and temperature reaches to 25oC is-
1. 20.6 atm
2. 12.6 atm
3. 15.8 atm
4. 2.8 atm
A mixture of CO and CO2 is found to have a density 1.5 g/litre at 30oC and 730 mm. The composition of the mixture is-
1. CO: 44%; CO2 = 56%
2. CO: 32%; CO2 = 68%
3. CO: 70%; CO2 = 30%
4. CO: 9%; CO2 = 91%
A collapsed polythene bag of 30 litre capacity is partially blown up by the addition of 10 litre of N2 at 0.965 atm at 298 K. Subsequently, enough O2 is pumped into bag so that at 298 K and external pressure of 0.990 atm, the bag contains full 30 litre. The final pressure of O2 in the following experiment is-
1. 0.67 atm
2. 0.52 atm
3. 85 atm
4. 20 atm
A 40 mL of a mixture of H2 and O2 was placed in a gas burette at 18 oC and 1 atm pressure. A spark was supplied so that the formation of water was complete . The remaining pure gas had a volume of 10 mL at 18 oC and 1 atm pressure. If the remaining gas was H2 , what was the initial mole percentage of H2 in the mixture?
1. 25%
2. 50%
3. 35%
4. 75%
60 mL of a mixture of equal volume of Cl2 and an oxide of chlorine was heated and then cooled back to the original temperature. The resulting gas mixture was found to have volume of 75 ml. On treatment with caustic soda solution, the volume contracted to 15 ml. Assume that all the measurements are made at same T and P. Deduce the simplest formula for oxide of Cl2. The oxide of Cl2 on heating decomposes quantitatively to O2 and Cl2.
(1) ClO
(2) Cl2O
(3) ClO4
(4) Cl2O3
For the reaction, N2 O5 (g)→2NO2 (g) + 0.5 O2 (g),
Calculate the mole fraction of N2 O5 (g) decomposed at a constant volume and temperature if the initial pressure is 600 mm Hg and the pressure at any time is 960 mm Hg. Assume ideal gas behaviour.
(1) 0.4
(2) 0.2
(3) 0.1
(4) 0.5
An underwater bubble with radius of 5 cm at the bottom of tank , where the temperature is 5oC and pressure is 3 atm rises to the surface, where temperature is 25 oC and pressure is 1 atm. What will be the radius of bubble when it reaches to the surface?
(1) 7.4 cm
(2) 4.9 cm
(3) 9.4 cm
(4) 13 cm
Which is lighter than dry air?
(1) Moist air
(2)
(3)
(4)
In the van der Waals' equation, the constant ' a ' and ' b ' with temperature shows which trend:
(1) both remains same
(2) ' a 'remains same, ' b ' varies
(3) ' a 'varies, ' b ' remains same
(4) both varies
At relatively high pressure, van der Waals' equation reduces to:
(1) PV=RT
(2) PV=RT+a / V
(3) PV=RT+Pb
(4) PV=RT-a /
Joule-Thomson coefficient for an ideal gas is:
(1) zero
(2) +ve
(3) -ve
(4) none of these
All the three states i.e., the triple point for the equilibrium,
Ice:
(1) 3.85mm and 0.0981
(2) 4.58mm and 0.0098
(3) 760mm and 0
(4) none of the above
An ideal gas cannot be liquefied because
(1) its critical temperature is always above 0
(2) its molecules are relatively smaller in size
(3) it solidifies before becoming a liquid
(4) forces operating between its molecules are negligible
Boyle's law is applicable in:
1. isobaric process
2. isochoric process
3. isothermal process
4. adiabatic process
Average speed of the molecules of a gas in a container moving in one direction is:
1.
2.
3.
4. Zero
Air at sea level is dense. This a practical application of:
1. Boyle's law
2. Charles's law
3. Avogadro's law
4. Dalton's law
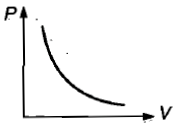
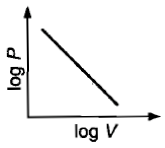
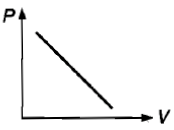
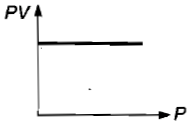
Which curve does not represent Boyle's law?
1. 
2. 
3. 
4. 
An open container is heatted from 300 K to 400 K then% of gas remain in the container
is:
1. 25%
2. 50%
3. 75%
4. 100%
Which relation is correct regarding N & NH?
1.
2.
3.
4.
The temperature at which the r.m.s. velocity of carbon dioxide becomes the same as that
of nitrogen at 21 C
1. 462C
2. 273K
3. 189C
4. 546K
20 L of diffuse through a porous partition in 60 s. volume of diffuse under
similar condition in 30 s will be
1. 12.14 L
2. 14.14 L
3. 18.14 L
4. 28.14 L
The compressibility factor a real gas at high presure is:-
1. 1
2.
3.
4.