Which of the following species absorb maximum energy in its HOMO-LUMO electronic transition?
Which of the following facts given is not correct?
| (I) | Bond length order : |
| (II) | have same bond order of |
| (III) | Bond order can assume any value including zero upto four |
| (IV) | and have same bond order for X - O bond (where X is central atom) |
1. I, II & III
2. I & IV
3. II & IV
4. I & II
and are converted to monocations and respectively. Which of the following is incorrect?
1. In , the bond weakens.
2. In , the bond order increases.
3. In , the paramagnetism decreases.
4. becomes diamagnetic.
In which of the following transformations, the bond order has increased and the magnetic behavior has changed?
The correct order of boiling point is :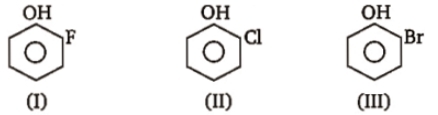
1. I > II >III
2. III > II > I
3. II > I > III
4. III > I > II
and both are covalent compounds but is polar whereas is non-polar. This is because :
1. Nitrogen atom is smaller than boron atom
2. N-F bond is more polar than B-F bond
3. NF is pyramidal whereas BF is planar triangular
4. BF is electron deficient whereas NF is not
Which of the following molecules will have polar bonds but zero dipole moment?
How many sp and sp-hybridised carbon atoms are present respectively in the following compound ?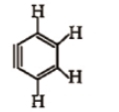
1. 4, 2
2. 6, 0
3. 3, 3
4. 5, 1
Which of the following species used both axial set of d-orbitals in hybridization of central atom ?
Which atom can have more than eight valence electrons when it is forming covalent bonds ?
1. H
2. N
3. F
4. Cl
Which molecule does not exist ?
The correct order of Cl-O bond order is
0.01 mole of is completely neutralized by 0.56 gram of KOH hence :
1. x = 3 and given acid is dibasic
2. x = 2 and given acid is monobasic
3. x = 3 and given acid is monobasic
4. x = 4 and given acid forms three series of salt
The shape of is :
1. Trigonal planar
2. Pyramidal
3. Bent T-shape
4. See-saw
The geometrical arrangement of orbitals and shape I are respectively :
1. trigonal bipyramidal geometry, linear shape
2. hexagonal geometry, triangular shape
3. triangular planar geometry, triangular shape
4. tetrahedral geometry, pyramidal shape
Which is the following pairs of species have identical shapes ?
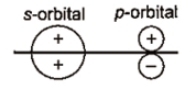
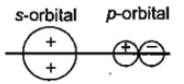
Which of the following leads to bonding ?
1. 
2. 
3. 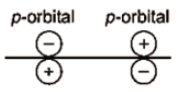
4. 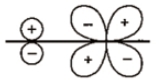
Which of the following overlap is incorrect (assuming Z-axis is internuclear axis) ?
(A) - Bond formation
(B) - Bond formation
(C) - Bond formation
(D) - Bond formation
(E) - Bond formation
(F) - Bond formation
1. A, B, C
2. C, F
3. B, E
4. B, C, D
Which of the following is not present in molecule ?
The incorrect order of lattice energy is :
The bond angles of \(\mathrm{NH}_3, \mathrm{NH}^+_4~\mathrm{and}~\mathrm{NH}^-_2\) are in the order :
| 1. | \(\mathrm{NH}^-_2> \mathrm{NH}_3>~\mathrm{NH}^+_4\) | 2. | \(\mathrm{NH}^+_4> \mathrm{NH}_3>~\mathrm{NH}^-_2\) |
| 3. | \(\mathrm{NH}_3> \mathrm{NH}^-_2>~\mathrm{NH}^+_4\) | 4. | \(\mathrm{NH}_3> \mathrm{NH}^+_4>~\mathrm{NH}^-_2\) |
The "O-N-O" bond angle is maximum in
1.
2.
3.
4.
Which of the following is incorrectly matched?
| Hybridisation | Geometry | Orbitals use | |
| 1. | \(sp^3d\) | Trigonal bipyramidal | \({s+p_x+p_y+p_z+d_{z^2}}\) |
| 2. | \(sp^3d^3\) | Pentagonal bipyramidal | \(\small[s+p_x+p_y+p_z+d_{x^2-y^2} +d_{z^2}+d_{xy}\) |
| 3. | \(sp^3d^2\) | Capped octahedral | \(s+p_x+p_y+p_z+d_{x^2-y^2} +d_{z^2}\) |
| 4. | \(sp^3\) | Tetrahedral | \(s+p_x+p_y+p_z\) |
Which of the following has a maximum 'Cl-O' bond order?
1.
2.
3.
4.
The correct statement regarding molecule is:
1. two bonds
2. molecules has 2 lone pair, 2 bonds and 2 bonds
3. two bonds
4. one and one bond