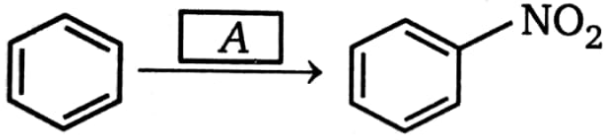
 , Best choice for A is:
, Best choice for A is:
1.
2.
3.
4.
Which of the following compounds will not give benzoic acid on oxidation with ?
1. 2. 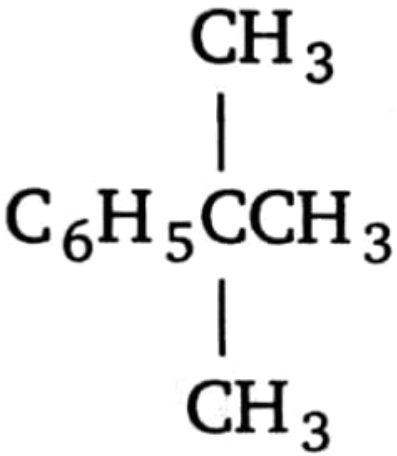
3. 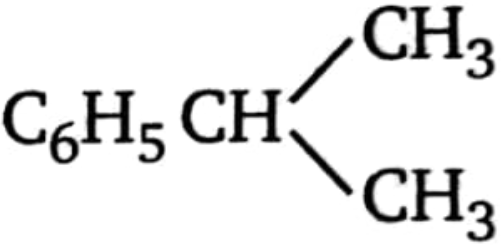 4.
4.
Which of the following is the electrophile that attacks the aromatic ring during Friedel-Crafts acylation?
1. 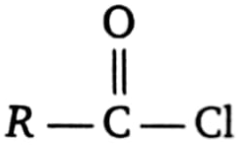 2.
2. ![]()
3. 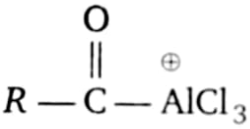 4.
4. 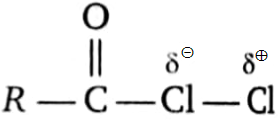
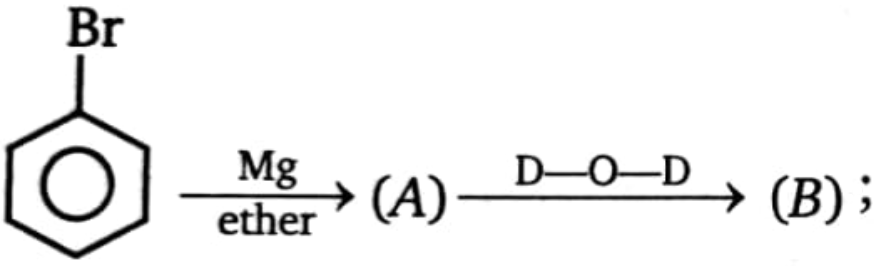 Product (B) is:
Product (B) is:
1. 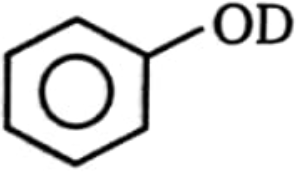 2.
2. 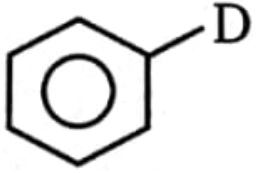
3. 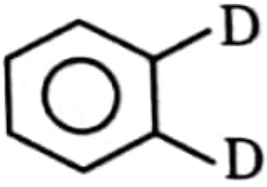 4. none of these
4. none of these
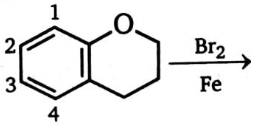 Substitution takes place at the position:
Substitution takes place at the position:
1. 1
2. 2
3. 3
4. Both (1) and (3)
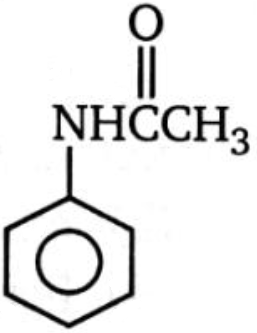
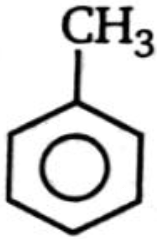
Which of the following compounds is more reactive than benzene toward ring bromination?
1. 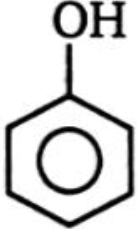 2.
2. 
3. 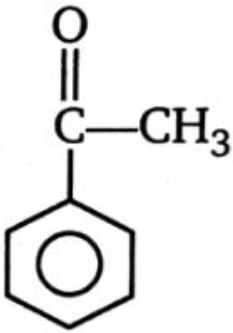 4.
4. 
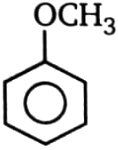
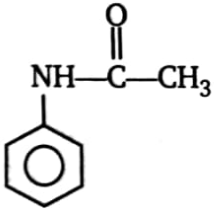
Which of the compound will not undergo Friedel Craft reaction?
1. 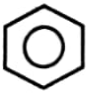 2.
2. 
3. 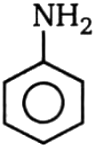 4.
4. 
Which of the following is least reactive for nucleophilic substitution reaction?
1.
2.
3.
4.
Which halide ion is the best nucleophile in dimethyl sulfoxide solution?
1. fluoride
2. chloride
3. bromide
4. iodide
Order of nucleophilicity in polar aprotic solvent?
1.
2.
3.
4.
Which of the following two isomers (A and B) will undergo substitution with faster and why?
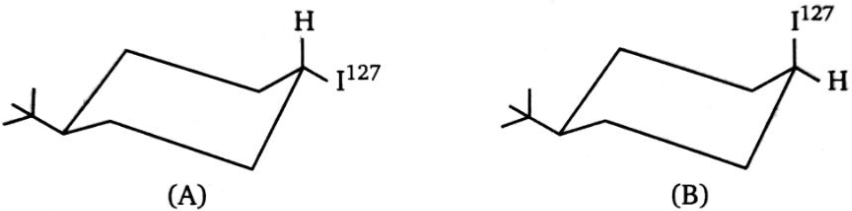
1. A will react faster because it is more stable than B
2. A will react fasts because it will yield more stable product and the transition state for both reactions is of the same energy
3. B will react faster because it is more stable than A.
4. B will react faster because it is less stable than A. The transition state for both reactions is of the same energy and it will yield more
stable product.
Which statement is true with respect to an reaction?
1. A good leaving group is a strong base
2. A good leaving group is a weak base
3. A leaving group must be negatively charged
4. A leaving group must be a halide
For the following halides, the order of rate of reaction with or rate of :
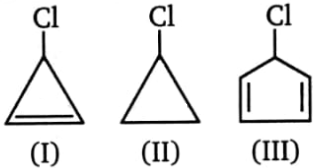
1. I > III > II
2. II > III > I
3. I > II > III
4. III > I > II
K1 and K2 are rates constant for the above reactions, correct relation is:
1. K1 = K2
2. K1 > K2
3. K1 < K2
4. K1 K2
Which of the following solvents is most suitable for this reaction?
1.
2.
3.
4. None of these
The rate of solvolysis of tert-butyl bromide will be maximum in which of the following solvents?
1.
2.
4.
4.
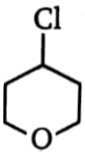
Which one of the following compounds will be most reactive for reactions?
1. 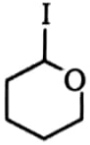 2.
2. 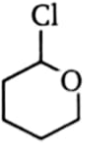
3. 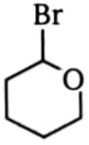 4.
4. 
When ethyl bromide is treated with moist main product is:
1. Ether
2. Ethanol
3. Aldehyde
4. All of the above.
Compare rate of reaction with or rate of reaction:
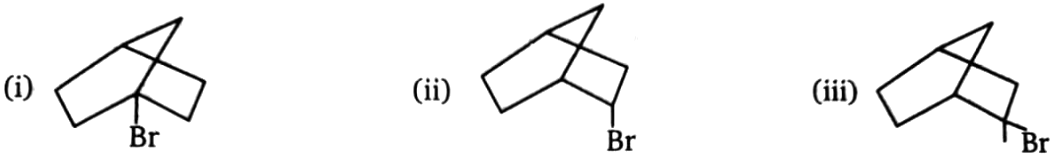
1. i > ii > iii
2. iii > ii > i
3. ii > iii > i
4. iii > i > ii
Compare rate of reaction with or rate of reaction:
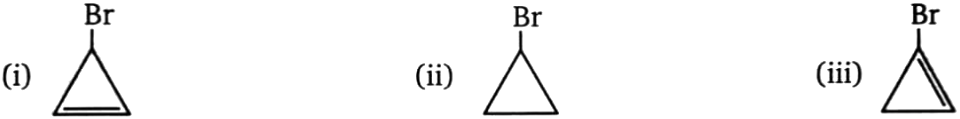
1. i > iii > ii
2. ii > iii > i
3. i > ii > iii
4. iii > i > ii
Which of the following is most reactive toward or rate of reaction?
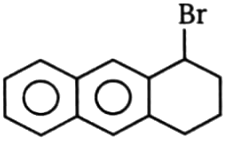
1.  2.
2. 
3. 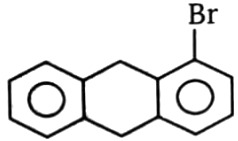 4.
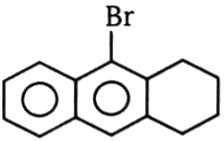
4. 
Compare rate of
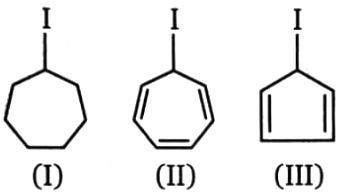
1. II > III > I
2. II > I > III
3. I > II > III
4. III > II > I
Rate of will not be negligible in:
1. 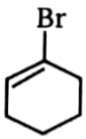 2.
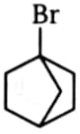
2. 
3. 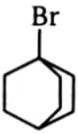 4.
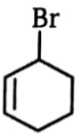
4. 
How many alkenes are formed by elimination of HBr from 2-bromo-2, 3-dimethyl hexane using a strong base such as sodium methoxide?
1. 1
2. 2
3. 3
4. 4
Which of the following is not reasonable for an elimination reaction?
1. ![]() 2.
2. ![]()
3. ![]() 4.
4. ![]()
The major product of the reaction 1,2-dibromobutane and excess is:
1. 2-bromo-1-butene
2. 1-bromo-1-butene
3. 1-butyne
4. 1,2-butadiene
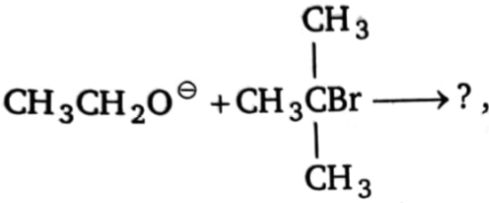 Product(s) of the reactio is/are:
Product(s) of the reactio is/are:
1. 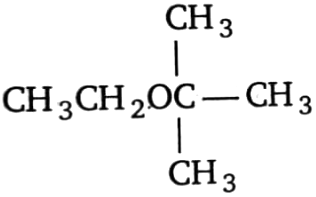 2.
2.
3. 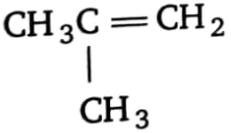 4. (1) and (2)
4. (1) and (2)
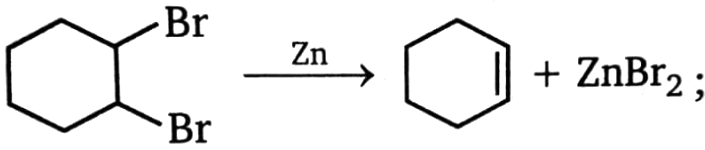 This reaction is a case of:
This reaction is a case of:
1. -elimination
2. -elimination
3. -elimination
4. None of these
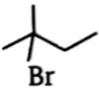
Which alkyl bromide will yield only one alkene in elimination?
1. 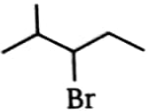 2.
2. ![]()
3. 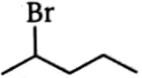 4.
4. 
In order to accomplish the following conversion, what reagent and conditions would be required?
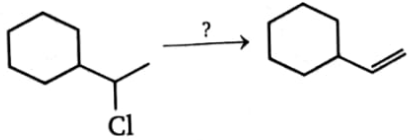
1. Cold sodium hydroxide
2. Hot conc. sodium hydroxide
3. Potassium t-butoxide and hear
4. Hot water
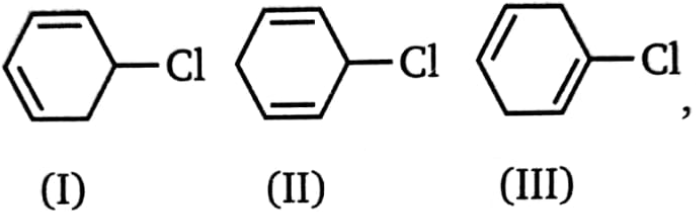 which is most easily dehydrohalogenated?
which is most easily dehydrohalogenated?
1. I
2. II
3. III
4. All with the same ease
Which of the following isomeric hexachlorocyclohexanes is least reactive in -dehydrochlorination on treatment with strong base?
1. 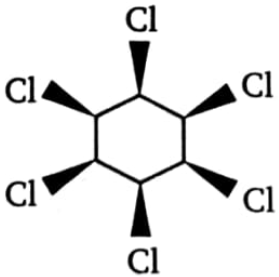 2.
2. 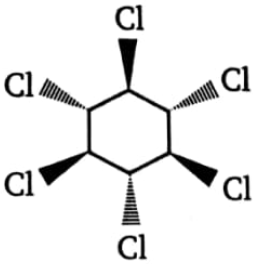
3. 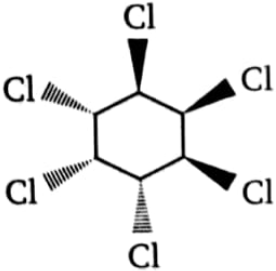 4. All three are equally reactive
4. All three are equally reactive